Markovnikov
ARTICLES
5/18/3000
Atoms Basics
Use this space to introduce yourself or your business to site visitors. Share who you are, what you do, and the purpose of this website. Feel free to include your background, experiences, and any unique aspects that set your business apart. Highlight your mission, values, and the benefits your customers can expect.
(Gaur, 2022)
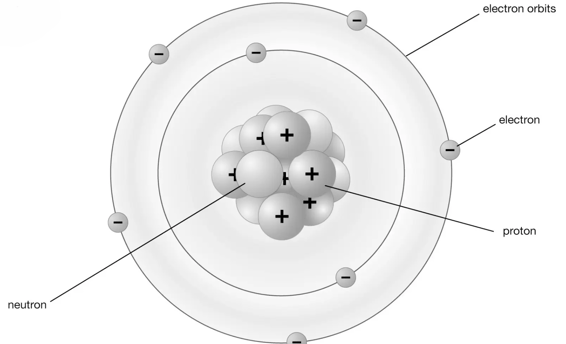
This is the traditional atom diagram—commonly referred to as the "Bohr model/diagram". It was presented the Danish physicist Niels Bohr, by which it was named after, and Ernest Rutherford in 1913.
This model depicts the atom as small, positively charged nuclei surrounded by electrons that travel in circular orbits around the nuclei—resembling planets orbiting a star.
Here, the atom is made up of three distinct particles:
Positively charged Protons
Negatively charged Electrons
Neutrons with a Neutral charge
Atomic Structure
Nucleons and Electrons
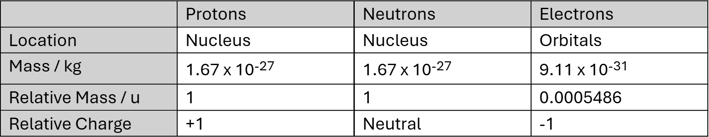
Every atom contains a small dense region consisting of nucleons—this is called the nucleus.
Hydrogen is the only stable element that only contains one type of nucleon—one proton and no neutrons (hydrogens stable isotope protium, to be specific). So, for the most part, normal atomic nuclei contain both types: positively charged Protons and neutrally charged Neutrons.
Since the nucleus contains positively charged protons and neutrally charged neutrons, it is considered to have an overall positive charge (no negative charges are present to cancel out the positive charges that are present). Atoms of an overall neutral charge are typically the most stable configuration, which is why, the number of negatively Electrons in an atom is generally equal to the number of protons in the nucleus, resulting in a neutral overall charge.
This balance occurs because electrons have an equal but opposite charge to that of a proton, and are thus electrostatically attracted to each other equally.
The majority of the mass of an atom is from within its nucleus as the mass of an electron is 1/1836th that of a nucleon.
A nucleon is either a proton or a neutron, considered in its role as a component of an atomic nucleus.
Definitions
Nuclei is plural for nucleus.
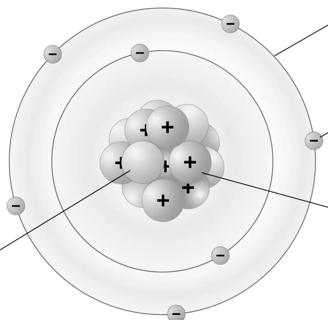
Electron Orbitals
The exact location of electrons are impossible to predict so we say that they are found in "electron clouds", which surrounds the nucleus of an atom.
the mass of an Electron is 1/1836th
Each orbit, or energy level, is quantized, meaning electrons can only occupy specific orbits at distinct energy levels. This model, while simplified and not fully accurate according to modern quantum mechanics, remains a foundational visualization in the teaching of atomic structure due to its historical significance and its ability to convey basic atomic concepts.
Atoms of an overall neutral charge are typically the most stable configuration, so if the number of electrons is different from the charge of the nucleus, the overall charge of the atom wouldn't be electrically neutral, thus, it would be considered an ion (The charge of regular nuclei is always positive, due to containing no negatively charged particles).
Ions, Isotopes & Radiation
Each orbit, or energy level, is quantized, meaning electrons can only occupy specific orbits at distinct energy levels. This model, while simplified and not fully accurate according to modern quantum mechanics, remains a foundational visualization in the teaching of atomic structure due to its historical significance and its ability to convey basic atomic concepts.
Reference List
Gaur, A. (2022). Bohr Atomic Model- Diagram, Formula, Postulates and Limitations. [online] adda247. Available at: https://www.adda247.com/school/bohr-atomic-model/.
f
f